Types of Licences, Registration and Approvals (with effect from 20 Feb 2023)

Note: You may refer to the list of controlled irradiating apparatus and radioactive materials, which are subjected to licensing by NEA, under Additional Licensing Information.
1 Each IR2 licence has its unique inventory list of irradiating apparatus and radioactive materials. Any update will be considered as a licence amendment.
2 Examples of industrial ultrasound apparatus ≥ 1,200 W:
- Ultrasonic cleaners
- Ultrasound imaging (for non-destructive testing purposes)
- Ultrasonic welding machines
3 Each N2 licence has its unique inventory list of apparatus contained within the site. Any update will be considered as a licence amendment.
4 An N3 licence is not required for registered medical practitioners or dentists if they have obtained the consent of both of the following persons to use a medical laser at a healthcare institution:
- the individual in charge of that healthcare institution;
- the licensee of the N2 licence granted in respect of that medical laser.
How to Apply for Radiation Licences
Types of Application Forms | Platform |
Apply for New, Renewal, Amendment and Cancellation Applications - Ionising Radiation Licences (e.g. IR1, IR2, IR3 Licences)
- Radiation Worker Registration (e.g. R1 Certificate)
- Non-Ionising Radiation Licences (e.g. N1, N2, N3 Licences)
| GoBusiness Singapore Portal |
Apply for approval to accumulate or transport radioactive waste | Contact NEA via NEA's online feedback form or myENV app |
Miscellaneous Forms - Entertainment Laser Checklist for Organising of Laser Event
- GIRO Application Form for Radiation Licences
- Medical Certificate Form for R1 Applications (MC-1)
- Medical Certificate Form for N3 Applications (MC-2)
| NEA ePortal |
Applicants will be required to log in using their Singpass accounts via business users5 or individuals6 to complete the online application forms.
Licences | Licence Holder | Login |
IR1, IR2, IR3, N1, N2 | Organisation (UEN) | Singpass via Business Users |
R1, N3 | Individual (NRIC, FIN, Passport number) | Singpass via Individuals |
5 Corppass is the authorisation system for entities to manage digital service access of employees who need to perform corporate transactions. Plese check that your organisation's Corppass administrator has granted you access to "GoBusiness Portal", which is different from "LicenceOne". With effect from 11 Apr 2021, the login process for Corppass is simplified to verify the user identity via Singpass for business users first, before the user can proceed to access and transact with government digital services. For more information, please refer to the FAQ within the Corppass website: www.corppass.gov.sg.
6 PRs and foreigners holding certain passes are eligible for Singpass and should register for a Singpass account. For more information, please see the Singpass website: www.singpass.gov.sg/register/instructions.
You may visit the “Help Topics” on the GoBusiness Singapore Portal for more information on how to submit an application. Should applicants require further assistance on submission of applications, such as those listed in the table below, please contact the relevant parties for support.
Issues with application | What should I do? |
- Unable to log in to your Singpass via Business Users or Individuals/GoBusiness account
- Unable to proceed to the next page of the application
- Application page loaded unsuccessfully
- Unable to make licence fee payment
- Unsure how to navigate the GoBusiness application form
| Please contact
AskGoBiz@crimsonlogic.com.sg for assistance. |
- Unsure of what information to provide within the application
- Unsure of your existing radiation licence number
- Unsure of which licence to apply for
- Unable to find your existing licence to amend, renew or cancel
| Please contact RPNSG through NEA's online feedback form or myENV app. |
For import and export licences (IR4A/B, IR5A/B/C, N4A/B): Please refer to Information on Licences to Import or Export Irradiating Apparatus or Radioactive Materials.
Information on New Licence Application and Approval
The table below shows the list of key licence- or approval-specific information and supporting documents that you will need to complete your application and the application fee for each licence or approval type. NEA may request for the submission of other relevant information or documents.
Licence or Approval Type | Information Required/Supporting Documents | Application/Annual Fee (per application or licence, unless otherwise stated) |
IR1, N1 | - Details of apparatus and radioactive materials such as
- [For both apparatus and radioactive materials] Name of manufacturer, country of manufacture and model
- [For apparatus only] Maximum voltage, maximum current
- [For radioactive materials only] Radionuclide, maximum radioactivity, form and nature of substance
- For radioactive materials only,
- Official document from your overseas supplier stating that they will accept the return of disused sources for proper disposal
- Official document to your customers stating that your organisation will accept the return of disused sources for proper disposal
- [For manufacturing of radioactive materials only] Radiation Protection Plan; information on radioactive waste generation and disposal/decommissioning plan
- Address of storage location (for IR1)/operation location (for N1)
- Brochures, catalogues or data sheets showing the technical specifications of the apparatus or radioactive materials
- [For laser apparatus] Manual showing laser safety instructions
- [For apparatus or radioactive materials to be used on human beings] Registration approval or authorisation from the Health Sciences Authority (HSA) for medical devices intended for local sale
| For IR1:
- $210 for first year
- $210 annual fee for subsequent years
For N1:
- $52.50 (3 months)
- $105 (6 months)
- $157.50 (9 months)
- $210 (1 year)
- $420 (2 years)
|
IR2 | - Particulars of supplier (name, address, IR1 licence number if local supplier)
- Details of apparatus and radioactive materials such as
- [For both apparatus and radioactive materials] Name of manufacturer, country of manufacture, model and serial number (note: If the serial number cannot be confirmed at time of application, applicant may indicate “TBC”)
- [For apparatus only] Maximum voltage, maximum current
- [For radioactive materials only] Radionuclide, maximum radioactivity, date as at stated radioactivity, form & nature of substance
- Storage location address
- Brochures, catalogues or data sheets showing the technical specifications of the apparatus or radioactive materials
- Floor plan showing position of apparatus or radioactive materials within controlled area and areas surrounding the controlled area
- Radiation Protection Plan
- [For accelerators and proton beam only] Decommissioning plan
- [For radioactive materials only] Official document from your supplier stating that they will accept the return of disused sources for proper disposal (if applicable)
- The approximate date of commissioning of the apparatus or radioactive materials. This will facilitate the arrangement of the pre-licensing inspection
Note:
- [For apparatus only] You may apply for the IR2 licence once the sales/purchase is confirmed. The pre-licensing inspection can only be conducted after installation and the apparatus should not be energised before the inspection unless authorisation is given.
- [For radioactive materials only] An authorisation letter will be issued to the applicant to temporarily possess the radioactive materials to facilitate the conduct of the pre-licensing inspection for IR2 licence.
| For first year: - $155 per irradiating apparatus
- $155 for at least one (a) irradiating apparatus containing radioactive material, or (b) radioactive material
For subsequent years: - $155 annual fee per irradiating apparatus
- $155 annual fee for at least one (a) irradiating apparatus containing radioactive material, or (b) radioactive material
|
N2 | - Particulars of supplier (name, address, N1 licence number if local supplier)
- Details of apparatus such as name of manufacturer, make/model and serial number
- Address of storage location
- Brochures, catalogues or data sheets showing the technical specifications of the apparatus
- [For laser apparatus] Manual showing laser safety instructions
- [For MRI apparatus] Detailed SOP, QA checklist, site map of location of use with 5-G lines indicated, quench exhaust line and photo of nameplate
- [For apparatus to be used on human beings] Registration approval or authorisation from the Health Sciences Authority (HSA) for medical devices
| - $155 per apparatus for first year
- $65 annual fee per apparatus for subsequent years
|
R1 | - Medical Certificate Form for R1 Applications (MC-1)
- Documentary proof of relevant training/qualifications
- [For medical doctors and dentists] Singapore Medical Council/Singapore Dental Council registration number
- [For veterinarians] Animal and Veterinary Service licence number
- [For medical diagnostic radiographers and radiation therapists] In-principle approval for registration or registration certificate with the Allied Health Professional Council (AHPC)
| - $105 for first year
- $50 annual fee for subsequent years
|
N3 | - Medical Certificate Form for N3 Applications (MC-2)
- Documentary proof of relevant training/qualifications (e.g. Basic Laser Safety Course certificate)
| - $26.25 (3 months)
- $52.50 (6 months)
- $78.75 (9 months)
- $105 (1 year)
- $210 (2 years)
|
IR3 | - Transport Emergency Response Plan
| - $155 for first year
- $155 annual fee for subsequent years
|
Approval to accumulate radioactive waste | - Type of radionuclide
- Quantity and its respective activities
- Radiation Protection Plan
- Storage location
- Floor plan
- Emergency preparedness and response plan
| $600 per annum |
Approval to transport radioactive waste | - Type of radionuclide
- Quantity and its respective activities
- Radiation Protection Plan
- Destination of transport
- Transport plan
- Emergency preparedness and response plan
| $600 |
Information on Amendment, Annual Fee Payment, Renewal and Cancellation of Licences
It is the responsibility of the licence or certificate holders to manage their licences or certificate. Licence or certificate holders must, where applicable: –
- renew their licences timely by submitting renewal applications at least one month before the licence expiry date;
- pay the annual fee for their licences or certificate by the anniversary date;
- ensure that licence details such as storage location and list of apparatus/radioactive materials are accurate and updated;
- update the customer profile timely when there is a change in point of contact (e.g. name, contact number and e-mail address);
- update the worker management timely when there is an addition or removal of radiation workers; and
- obtain authorisation from NEA to dispose of or decommission apparatus/radioactive materials and cancel licences that are no longer required.
You will be able to see the record(s) of all radiation licences (except import/export licences applied through TradeNet) tied to your UEN or individual identification number when you log into the GoBusiness Singapore Portal. Please select the relevant licence and submit all licence amendment, renewal and cancellation requests through the GoBusiness Singapore Portal.
As email notifications (e.g. application approval, payment notice, or reminders, etc.) will be sent to the contact email address, please ensure that the contact email address is up to date. The contact information (e.g. contact person name, contact number and email address, etc.) can be updated in the Settings tab on the GoBusiness Singapore Portal or via Customer Profile and Worker Management on NEA ePortal.
Amendment | Type of Amendment | How to request for amendment | Fee | Addition or removal of apparatus to existing N1 licence | Submit application to amend licence on GoBusiness Singapore Portal | Amendment fee of $25 per licence | Addition of apparatus to existing IR1 licence | Addition of new radioactive materials to existing IR1 or IR2 licences7 | Amendment of apparatus or radioactive materials details (e.g. storage location) in existing licence (IR1, IR2, N1, N2) | | Removal of apparatus from existing N2 licence | Addition or removal of QPs within the IR1 or IR2 licences | Addition of new apparatus to IR28 and N2 licence9 | Submit application to amend licence on GoBusiness Singapore Portal | Amendment fee of $155 per irradiating apparatus | Change in organisation name (without change in UEN) | Submit application to amend licence on GoBusiness Singapore Portal | Amendment fee of $25 per licence | Removal of apparatus or radioactive materials from existing IR1 and IR2 licences10 | Submit application to amend licence on GoBusiness Singapore Portal | No charge | Change in mailing/billing address or point of contact(s) (e.g. name, contact number and e-mail address) | Update on NEA ePortal > Customer Profile and Worker Management. | No charge |
7 Relevant supporting documents are required. Please see "Information on New Licence Application and Approval" (above) and "Cancellation" (below) for information on supporting documents required for addition and removal, respectively.
8 For addition of new apparatus to IR2 licence, licensees will have to submit an IR2 licence amendment application with the relevant support documents mentioned under the “Information on New Licence Application and Approval” (above).
9 For addition of new apparatus to N2 licence, if there is no need to amend details of existing apparatus in the licence, please do not fill up section 4 “Amendment Description (Please include purpose/reason)” or there will be an additional $25 charged for the amendment fee.
10 Please refer to the requirements under “Cancellation” for removal of apparatus or radioactive materials from existing IR1, IR2, N1and N2 licences. |
Annual Fee Payments | For IR1, IR2, IR3, N2 licences and R1 certificates, a payment notice will be sent via email to the customer profile contact person 60 days before the annual payment due date for annual fee payment. Annual fee must be paid before the anniversary date stated in the licence (e.g. if anniversary date is on 30 Apr, annual fee payment due date is on 29 Apr of each year). Payment methods for the annual fee payment can be found below under section on “Payment Methods for Licence Application and Annual Fee”.
Please note that for IR2 and N2 licences, item newly added to the licence within 60 days prior to the annual payment due date will not be included in the annual fee.
If the annual fees are not paid (for licensees without GIRO or government payment arrangement) by the annual payment due date or GIRO deduction is unsuccessful (for licensees with GIRO arrangement), enforcement action may be taken including the suspension or revocation of the licence or certificate. When a licence or certificate is revoked, re-application of new licence or certificate will incur the fees for new application.
Separately, individuals engaging in non-destructive testing (NDT) radiation work (also known as industrial radiography) using Cobalt-60, Iridium-192 or Selenium-75 are required to undergo a medical examination and submit a new medical certificate (including full blood examination) annually to NEA. The medical certificate form (MC-1) can be found on the NEA ePortal: Miscellaneous Forms.
With the decoupling of personal dose monitoring services from licence fees with effect from 20 Feb 2023, existing subscribers will have to renew their subscription and pay for the dose monitoring service at the rate of $15.75 per month (inclusive of GST) before their subscription expires. More details can be found in the FAQs on new eServices. |
Renewal | Renewal applications can be submitted on the GoBusiness Singapore Portal starting 90 days from the licence expiry date. All licence renewal applications should be submitted at least 30 days prior to the expiry of the existing licences. If you are unable to find your licence under your account, unable to select it for renewal, or if your licence is no longer active on GoBusiness Portal, please contact NEA for assistance. Note: Applicants will not be able to make any amendments to the existing licence as part of the renewal application. |
Cancellation | Licence cancellation applications can be submitted on the GoBusiness Singapore Portal.
[For irradiating apparatus] IR1, IR2, N1 and N2 licence holders must cancel their licences after disposal of the apparatus. For apparatus that may have activated components (e.g. Linear Accelerators (LINAC), proton beam, cyclotrons, synchrotron, etc.) and apparatus containing radioactive material, licensees must obtain authorisation for disposal of the apparatus before carrying out the disposal.
[For radioactive materials] IR1 and IR2 licence holders must obtain authorisation for disposal of the material before carrying out the disposal.
Licensees will be required to submit the licence cancellation application on GoBusiness Singapore Portal and provide supporting documents such as export/repatriation/disposal/decommissioning/transfer documentation, depending on the type of irradiating apparatus they possess. The serial number of the irradiating apparatus should be clearly stated in the document(s). RPNSG officers may contact your organisation to arrange an inspection for verification. |
Payment Methods for Licence Application and Annual Fees
The following payment modes are accepted for licence applications and annual fee payment. Please note that cheque payment is no longer accepted. Fees paid or submitted are non-refundable.
Payment Mode | Remarks |
e-Payment | e-Payment can be made on: - GoBusiness Singapore Portal via VISA, MasterCard and American Express, when submitting an application on the portal; or
- NEA ePortal > Payment > One Stop Payment via eNETS, VISA and MasterCard, for other payments.
|
AXS | AXS is only available for annual fee payment.
Please provide the application number (within the payment notice sent to the customer profile contact person) as the reference number when making payment through AXS. |
GIRO Payment | (i) Billing Customers with Existing GIRO arrangement with RPNSG: For Licence Application
Applicants should select GIRO as the payment mode when submitting the application on the GoBusiness Singapore Portal and select/indicate the GIRO reference number in the application form. The GIRO reference number can be found in the letter confirming the set-up of the GIRO account or on previously issued GIRO invoices.
For individuals (i.e. N3 or R1 applicants) using their organisation’s GIRO reference number for payment, they should obtain authorisation from their organisation to do so prior to making payment. For Annual Fee Payment
GIRO deduction will take place within the 2 weeks after the annual payment due date. Please ensure that sufficient funds are available in your account for the deduction to take place.
For R1 registrants, if you wish to pay annual fees using your employer's existing GIRO account with NEA for radiation protection licences, please contact NEA to set up the arrangement at least 3 months before the next anniversary date of your licence.
If you do not wish to pay subsequent annual fees via GIRO, please inform NEA at least 3 months before the next anniversary date. (ii) Billing Customers with No Existing GIRO arrangement with RPNSG: For Licence Application or Annual Fee Payment
For customers who wish to set up GIRO arrangement with RPNSG, please complete and submit the GIRO application form found on the NEA ePortal: Miscellaneous Forms at least 3 months before the application/expiry/anniversary date. Setting up of a GIRO account may take 4 to 8 weeks. |
Government
e-Invoicing | This is applicable only for government organisations using the e-invoicing system.
As part of the transition, NEA has endeavoured to update government customer profiles to set the annual fee to be paid by government payment mode. However, government employees who are R1 registrants will need to log into NEA ePortal to update their customer profiles to update the annual fee payment mode and other details, if necessary. Government entities should also log into NEA ePortal to ensure that profile details are correct. For Licence Application
Applicants should select government payment options as the payment mode when submitting applications on the GoBusiness Singapore Portal. For Annual Fee Payment
If you have indicated to NEA that the annual fee will be paid by a government entity through government payment modes at least 3 months before the anniversary date, an invoice will be issued to government entity within 1 week from the annual payment due date.
To add or remove government payment mode for subsequent annual fee payments for issued licences or certificate, please log into NEA ePortal to update your customer profile at least 3 months before the next anniversary date of your licence/certificate.
Please note that payment through government payment modes will have to be made within the 30-day credit term. |
Processing of applications
Duly completed licence application forms that are submitted with complete information and payment will typically be processed within 4 weeks upon receipt of the submission. An email notification will be sent to the contact email address when the application is processed or approved. Issued licences will be sent via email in PDF form. Licence applications that require pre-licensing inspections and/or licence applicants who are instructed to sit for a qualifying test may require a longer period of time of about 4 to 8 weeks or more.
Overview of licence application process:
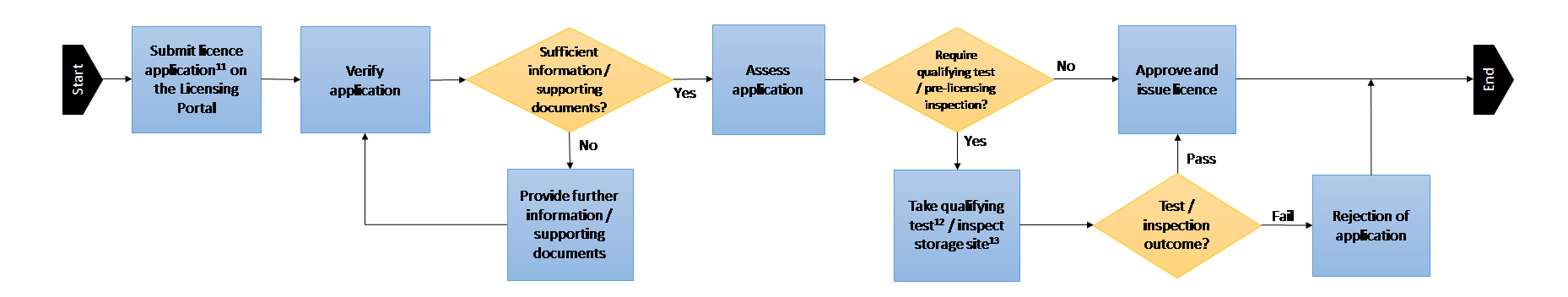
11 To facilitate the arrangement of the pre-licensing inspection, please indicate the following information in your licence application for IR2 licence:
| Item type | Information to be included in licence application |
| Ionising irradiating apparatus | - Indicate the approximate date of commissioning of the equipment on the licence application form.
Note: You may apply for the IR2 licence once the sales/purchase is confirmed. The pre-licensing inspection can only be conducted after installation and the apparatus should not be energised before the inspection unless authorisation is given. |
| Radioactive materials or ionising irradiating apparatus with radioactive materials | - Indicate the approximate date of commissioning of the radioactive materials or apparatus with radioactive materials.
Note: An authorisation letter will be issued to the applicant to temporarily possess the radioactive materials to facilitate the conduct of the pre-licensing inspection. |
12 Failure to pass the Qualifying Test on two consecutive attempts will result in the application being rejected.
13 RPNSG officers will conduct pre-licensing inspections to ensure regulatory compliance prior to the issuance of the licence. If any non-compliance is found during the inspection, applicants will generally be given reasonable time to make corrections. Continued failure or inability to meet regulatory requirements will result in the application being rejected.
Business activities/dealings or work with irradiating apparatus and radioactive materials should only commence after the appropriate licence(s) are obtained. Please note that it is an offence to manufacture, possess for sale, deal in, possess for use, handle or transport any controlled apparatus/radioactive materials without first obtaining the relevant licence(s).
All licence applications submitted are subjected to approval from NEA. NEA may at its discretion reject any application that is inadequate, lacking or unsubstantiated.